Arunika Gunawardena
 |
PROFESSOR |
|||
| Teaching & Research programmed cell death, apoptosis, lace plant, Aponogeton madagascariensis, plant development, cell biology, perforation formation, caspases, ethylene, developmental PCD, anthocyanin, reactive oxygen species, live cell imaging, autophagy
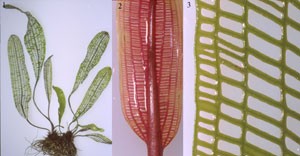
Aponogeton madagascariensis (lace plant) are the only vascular plants that form perforations by PCD during leaf development. Lace plant is native to Madagascar where it grows in river habitats as a submerged aquatic. Mature leaf blades are highly unusual in that they are perforated with holes that extend through the blade, forming an open lattice pattern. Perforations are positioned equidistantly between the longitudinal and transverse veins, and are large and rectangular near the midvein, but smaller and rounder near the margin. Immature leaves are rolled longitudinally, but unfurl as they expand from the apical region of the corm-like tuber to form a flat leaf blade with a simple shape. Young leaves are red in color due to anthocyanin which disappears as they mature (Figures 1-3).
The accessibility and predictability of perforation formation in aquatic lace plant provides an extremely tractable system in which to study the process of PCD, as well as its developmental control. Although some of the steps of cell death execution have been identified in this unique case of developmentally-regulated PCD in plants, little is known about the developmental cues, signaling pathways, or molecular regulation of the PCD process during perforation formation. Therefore, the objective of my research is to achieve a fundamental understanding of developmentally regulated PCD using lace plant leaf morphogenesis as a model system.
|
||||
|
1. Rantong, G., van der Kelen, K., van Breusegem, F., and Gunawardena, A.H.L.A.N. (2016). International Journal of Plant Sciences. Identification of differentially expressed genes during lace plant leaf development. International Journal of plant Sciences (cover image). 177(5):419–431. 2. Dauphinee, A.N.D., Lacroix, C., and Gunawardena, A.H.L.A.N. (2015). A comparison of the early developmental morphologies of Aponogeton madagascariensis and A. boivinianus. Botany. 93: 783–791. 3 Rantong G, Evans R, Gunawardena A.H.L.A.N. (2015). Lace plant ethylene receptors, AmERS1a and AmERS1c, regulate ethylene-induced programmed cell death during leaf morphogenesis. Plant Molecular Biology (cover image). 89:215–227. 4. Rantong, G. and Gunawardena A.H.L.A.N. (2015). Programmed Cell Death: Genes involved in signaling, regulation and execution in animals and plants. Botany 93: 193–210 5. Dauphinee, A.N.D., Warner, T.S. and Gunawardena A.H.L.A.N. (2014). A comparison of induced and developmental cell death morphologies in the lace plant (Aponogeton madagascariensis). BMC Plant Biology 14: 389 doi:10.1186/s12870-014-0389-x 6. Lord, C., Dauphinee, A., Watts, R. and Gunawardena, A.H.L.A.N. (2013). Unveiling Interactions among Mitochondria Caspase-Like Proteases and the Actin Cytoskeleton during Plant Programmed Cell Death (PCD). PLOS ONE. http://dx.plos.org/10.1371/journal.pone.0057110 7 Dauphinee, A., Wright H., Rantong, G., and Gunawardena, A.H.L.A.N. (2012). The involvement of ethylene in programmed cell death and climacteric-like behaviour during the remodelling of lace plant leaves. Botany. doi: 10.1139/b2012-093 8. Wertman, J., Lord, C., Dauphinee, A.N., and Gunawardena, A.H.L.A.N. (2012). The pathway of cell dismantling during programmed cell death in lace plant (Aponogeton madagascariensis) leaves. BMC Plant Biology. DOI: 10.1186/1471-2229-12-115. This paper received a Press release and was highly accessed. http://www.biomedcentral.com/presscenter/pressreleases/20120725 9. Lord, C. and Gunawardena, A.H.L.A.N. (2012). Programmed cell death in C. elegans, mammals and plants. European Journal of Cell Biology (cover image). 91: 603-613. doi:10.1016/j.ejcb.2012.02.002. 10. Lord, C. and Gunawardena, A.H.L.A.N. (2012). The Lace Plant: A Novel Model System to study plant proteases during developmental Programmed Cell Death in vivo. Physiologia Plantarum. 145:114-120. 11. Lord, C, Wertman, J., Lane, S. and Gunawardena, A.H.L.A.N. (2011). Do mitochondria play a role in remodelling lace plant leaves through programmed cell death?” BMC Plant Biology doi:10.1186/1471-2229-11-102. 12. Carter, J. and Gunawardena, A.H.L.A.N. (2011). Regeneration of the aquatic monocot Aponogeton madagascariensis (lace plant) through callus induction. Aquatic Botany 94: 143–149. 13. Lord, C., and Gunawardena, A.H.L.A.N (2011). Environmentally induced programmed cell death in leaf protoplasts of Aponogeton madagascariensis. Planta 233: 407-421. 14. Lord, C., and Gunawardena, A.H.L.A.N (2010). Isolation of leaf protoplasts from the submerged aquatic monocot Aponogeton madagascariensis. The Americas Journal of Plant Science and Biotechnology 4 (Special issue 2), 6-11. 15. Elliott, A. and Gunawardena, A.H.L.A.N. (2010). Calcium inhibition halts developmental programmed cell death in the lace plant, Aponogeton madagascariensis? Botany 88: 206-210. 16. Wright, H., DeLong, J., Harrison, P, Gunawardena A.H.L.A.N., and Prange, R. (2010). The effect of temperature and other factors on chlorophyll a fluorescence and the lower oxygen limit in apples (Malus domestica). Postharvest Biology and Technology 55: 21–28. 17. Wright, H., van Doorn W.G, Gunawardena, A.H.L.A.N. (2009). In vivo study of developmental programmed cell death using the lace plant (Aponogeton madagascariensis; Aponogetonaceae) leaf model system. American Journal of Botany (cover image). 96(5): 865-876. 18. Gunawardena A.H.L.A.N. (2008) Programmed cell death and tissue remodeling in plants. Journal of Experimental Botany 59: 445 – 451. 19. Urquhart, W., Gunawardena, A.H.L.A.N., Moeder, W., Ali, R., Berkowitz, G.A., and Yoshioka, K. (2007). The chimeric cyclic nucleotide-gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner. Plant Molecular Biology 65: 747-761. 20. Gunawardena, A.H.L.A.N., Greenwood, J.S., and Dengler, N.G. (2007). Cell wall degradation and modification during programmed cell death in lace plant, Aponogeton madagascariensis (Aponogetonaceae). American Journal of Botany (cover image) 94(7): 1116–1128. 21. Gunawardena, A.H.L.A.N., and Dengler, N.G. (2006). Alternative modes of leaf dissection in monocotyledons. Botanical Journal of Linnean Society 150: 25-44.
|

 y research interest is programmed cell death (PCD) in plant development. The development of complex leaf shape through perforation formation is a unique and fascinating use of developmental PCD. Several Monstera species (Araceae) and a single species of the distantly related Aponogeton family,
y research interest is programmed cell death (PCD) in plant development. The development of complex leaf shape through perforation formation is a unique and fascinating use of developmental PCD. Several Monstera species (Araceae) and a single species of the distantly related Aponogeton family,