Study of NMC positive electrode materials
Thermal characteristics of Li-ion cells are one of the most significant aspects for safer battery design. Accelerating rate calorimetry (ARC) is used to characterize the reactions between charged electrodes and electrolytes at elevated temperatures. ARC tests are conducted under adiabatic conditions which allows the self-heating rate of tested samples to be strictly a function of the intrinsic heat generating reactions1. The computer tracks the self-heating rate of tested samples by heating up the jacket to maintain the same temperature between the sample and the jacket. Figure 1(a) shows the instrument used for ARC tests and 1(b) shows the typical self-heating rate vs. temperature curve including a simple illustration about the data. Figure 2 shows ARC results for reactions between some popular positive electrodes and traditional electrolyte (1M LiPF6 in EC/EMC 3/7). For details, please consult reference 2.
Figure 1
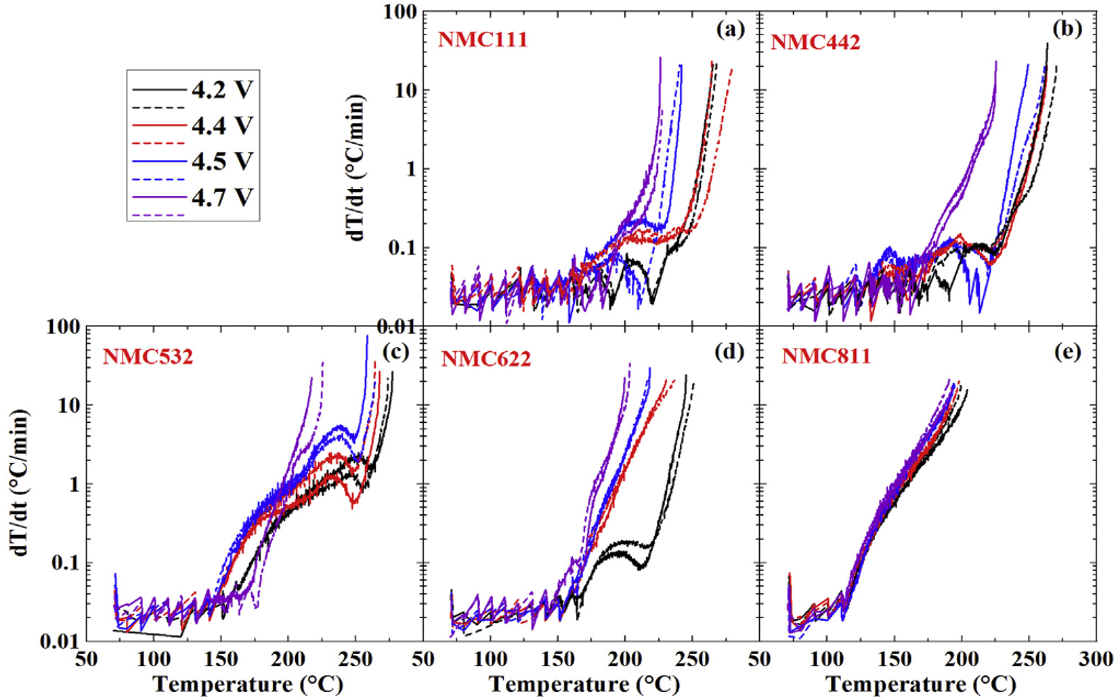
Figure 2. The self-heating rate vs. temperature for the reactions between electrolyte (1M LiPF6 in EC/EMC 3/7) and (a) NCM111, (b) NMC442, (c) NMC532, (d) NMC622 and (e) NMC811 at different upper-cut-off voltages. These data shows that NMC811 will be very difficult to make safe in Li-ion cells.
References:
- D. D. MacNeil and J. R. Dahn, J. Phys. Chem. A, 105, 4430–4439 (2001).
- L. Ma, M. Nie, J. Xia, and J. R. Dahn, J. Power Sources, 327, 145–150 (2016).
